Welcome to the Welte lab!

Research Overview
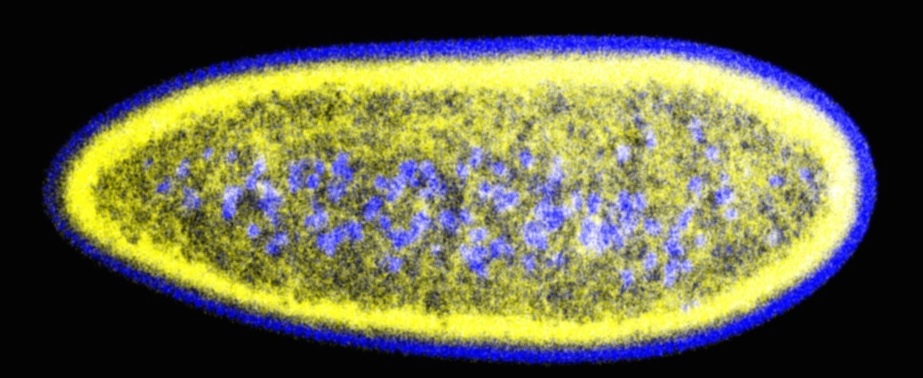
Lipid droplets (yellow) and nuclei (blue) in early Drosophila embryos
Cells are highly dynamic systems, in which many components are in constant flux: these include molecules, supramolecular complexes, and even entire organelles. They are continually generated, reorganized, relocated, and destroyed. Intracellular trafficking is highly directed in space and carefully controlled in time. The Welte laboratory studies the molecular mechanisms and biological roles of two types of trafficking events: the exchange of proteins between lipid droplets, cytoplasmic fat storage organelles, and the nucleus, as well as the motor-powered transport of various cargoes along microtubules. We employ Drosophila models in which we can combine in vivo visualization with molecular genetics approaches. We dissect the molecular mechanisms of these trafficking events and determine the consequences of disrupted trafficking at the organismal level. Our research is very visual and generates striking images (see the examples on the research or movies page). Currently, we work in three general areas: the role of lipid droplets as sequestration sites for nuclear proteins, the mechanism by which a nesprin family member modulates motor-driven transport, and the temporal regulation of lipid-droplet motion.
Lipid droplets as protein sequestration sites
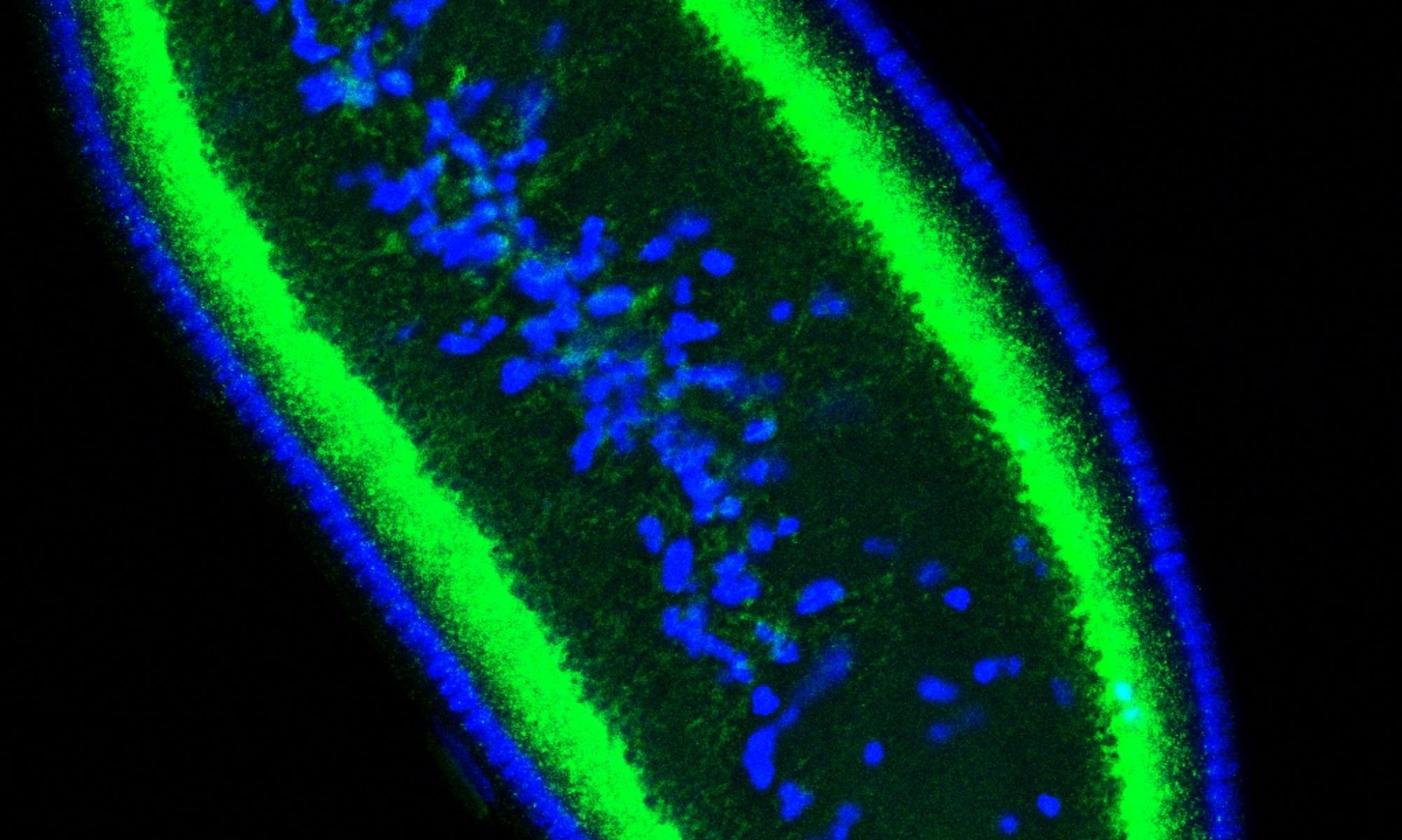
The variant histone H2Av (green) is present on lipid droplets and in nuclei
Lipid droplets are critical for lipid metabolism and energy homeostasis. We found that in Drosophila embryos lipid droplets also sequester massive amounts of certain histones (Cermelli et al., 2006), via the novel histone anchor Jabba (Li et al., 2012). Droplet-bound histones can be released and travel to the nuclei where they support chromatin assembly. In addition, droplet-bound histones mediate anti-bacterial defense (Anand et al., 2012). These studies revealed that in addition to their role in lipid metabolism lipid droplets also function as sites for regulated protein sequestration (Welte, 2007). For histones, such sequestration is important both for long-term storage (Li et al., 2012) and short-term buffering (Li et al., 2014). We are characterizing how Jabba recruits histones to lipid droplets, how the cell regulates histone release from droplets and their transfer to nuclei, and how droplet-bound histones contribute to innate immunity.
For information on current projects in the lab, please see our members page as well as our research topics page.